

The transition, from open surgery to multi-port laparoscopic surgery, and then to single-port laparoscopic surgery and even natural orifice transluminal endoscopic surgery (NOTES), has reduced patient trauma significantly while increasing the complexity of surgical operations. It is a prevailing trend to use robotic technology to enhance the intuitiveness and precision of surgical procedures.
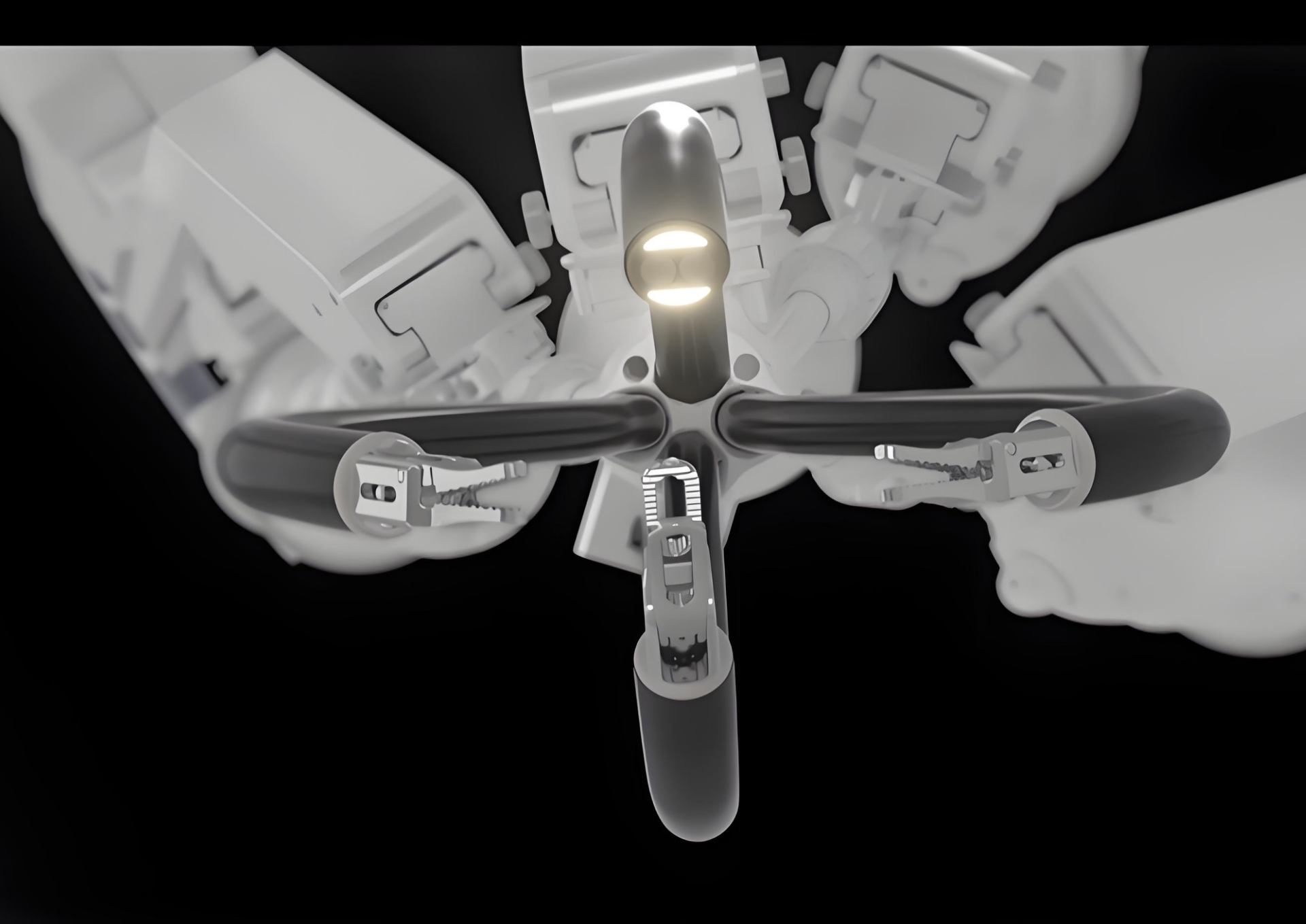
The SHURUI single-port surgical robot has been approved by the National Medical Products Administration of China, making it the first single-port surgical robot approved for use in both urology and gynecology surgery in China. The National Medical Products Administration (NMPA) commented highly on the SHURUI single-port robot: The surgical instruments of this product adopt internationally pioneering technologies with independent intellectual properties, which have technical advantages such as a wide range of motion, high payload capacity, and high reliability.




 Important Notes
Important Notes
The SHURUI Single-port Endoscopic Surgical System (SR-ENS-600), developed and produced by Beijing Surgerii Robotics Company Limited, has obtained the CE marking for urologic laparoscopic surgical procedures, gynecologic laparoscopic surgical procedures, general laparoscopic surgical procedures, and thoracoscopic surgical procedures. It is intended for use by trained surgeons in an operating room environment in accordance with the representative, specific procedures set forth in the User Manual.
Safety and Performance Information
Beijing Surgerii Robotics Co., Ltd. owns Surgerii®, SHURUI®, and other trademarks and may not be used without permission.