

At the National Children's Medical Center in Shanghai, a 6-month-old infant diagnosed with a malignant testicular tumor was successfully treated using the SHURUI® single-port surgical robot. This marked the 20th clinical procedure since the pediatric urology team at Shanghai Children's Medical Center adopted the domestically developed robotic platform earlier this year.
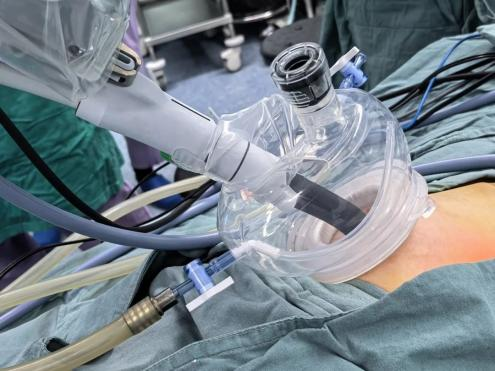
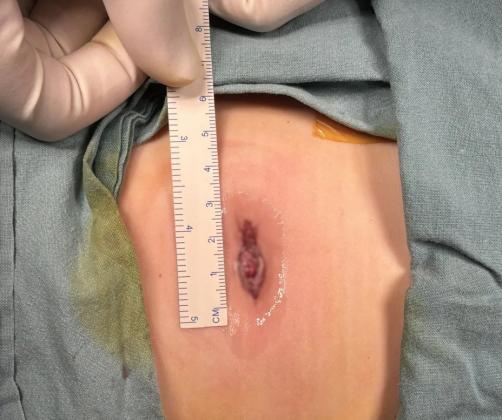
With just a 2.5 cm umbilical incision, the team precisely removed the tumor using snake-like instruments and 3D imaging guidance—minimizing trauma and preventing hematogenous spread. Total blood loss was under 3 ml, and the patient resumed normal activity within 24 hours.
The SHURUI® system offers sub-millimeter precision in exceptionally narrow surgical fields. With over a dozen pediatric procedure types explored in clinical studies, the platform is shaping a China-led solution for robotic surgery in children.
Shanghai Children's Medical Center is now expanding training infrastructure and national outreach to broaden access to safer, ultra-minimally invasive care for pediatric patients.

 Important Notes
Important Notes
The SHURUI Single-port Endoscopic Surgical System (SR-ENS-600), developed and produced by Beijing Surgerii Robotics Company Limited, has obtained the CE marking for urologic laparoscopic surgical procedures, gynecologic laparoscopic surgical procedures, general laparoscopic surgical procedures, and thoracoscopic surgical procedures. It is intended for use by trained surgeons in an operating room environment in accordance with the representative, specific procedures set forth in the User Manual.
Safety and Performance Information
Beijing Surgerii Robotics Co., Ltd. owns Surgerii®, SHURUI®, and other trademarks and may not be used without permission.